The Radiation Imaging: From Gamma-Rays to PET/SPECT
- Lentark
- Dec 2, 2024
- 22 min read

1. The Evolution of Medical Imaging
Medical imaging has transformed modern healthcare, offering non-invasive insights into the human body. From the serendipitous discovery of X-rays to the sophisticated MRI and PET systems of today, the journey of medical imaging is a testament to human ingenuity. Let’s explore how this remarkable field began and evolved.
1.1. The Birth of X-ray and Gamma Radiation Imaging for Medical Purposes
1895 - The Discovery of X-rays: Wilhelm Röntgen’s accidental discovery of "X-rays" revolutionized medicine by enabling us to view the inner workings of the human body. Röntgen received the first Nobel Prize in Physics in 1901 for his work. Tragically, the harmful effects of radiation were not yet understood, leading to premature deaths, including Röntgen and his colleagues.
1896 - The Phenomenon of Radioactivity: Henri Becquerel observed that uranium compounds emitted rays capable of fogging photographic plates. This laid the groundwork for understanding radioactivity, a crucial principle in medical imaging.
1.2. Mid-Century Innovations and the Digital Revolution
1930s - Radioactive Isotopes: Early uses of radionuclides focused on therapy and metabolic studies rather than imaging. Gamma-ray imaging with rectilinear scanners emerged in the 1950s, later replaced by detector arrays.
1970s - The Rise of Computed Tomography (CT): Building on Johann Radon’s 1917 mathematical principles, advances in computing power led to breakthroughs in CT and PET imaging. These technologies provided cross-sectional images, revolutionizing diagnostics.
1.3. Modern Era and Future Prospects
The 21st century is dominated by the "Big Four" in medical imaging: X-ray, radionuclide imaging, ultrasound, and MRI. However, innovations continue to expand the boundaries of imaging, integrating molecular-level analysis and advanced techniques like PET-CT and functional MRI.

2. Imaging Devices
Medical imaging devices are at the heart of nuclear medicine, where the distribution of radiopharmaceuticals in the body helps differentiate between normal and abnormal tissues. Two of the most significant advancements in this field are rectilinear scanners and scintillation (gamma) cameras.
2.1. From Rectilinear Scanners to Gamma Cameras
In the early 1950s, rectilinear scanners marked the beginning of imaging radionuclide distributions. These scanners utilized a NaI(Tl) (thallium-activated sodium iodide) crystal detector. While this technology provided point-by-point imaging, it was time-consuming and lacked the ability to visualize an entire area simultaneously.
To address these limitations, the gamma camera, also known as the Anger camera, was introduced in the late 1950s by Hal Anger. Unlike its predecessor, the gamma camera enabled simultaneous visualization of a larger area with higher spatial resolution, revolutionizing nuclear medicine imaging.
2.2. How Gamma Cameras Work
Gamma cameras detect the radiation emitted from radiotracers introduced into the patient’s body. These devices include:
A NaI(Tl) detector for sensing gamma rays.
Photomultiplier (PM) tubes to amplify signals.
A computer system for data acquisition and image processing.
The detector's sensitivity and resolution are influenced by its thickness. While thicker detectors increase sensitivity, they also reduce resolution due to higher probabilities of Compton scattering. Thus, a balance is achieved with thin detectors for better image clarity.

2.1. Photomultiplier Tube
The photomultiplier tube (PMT) plays a vital role in scintillation and gamma cameras by converting light photons into amplified electrical signals for precise imaging. When gamma rays interact with the scintillation crystal, they produce visible light flashes, which the PMT detects. Inside the PMT, these light photons strike the photocathode, releasing electrons through the photoelectric effect. The electrons are then multiplied through a series of dynodes, each amplifying the signal, before being collected at the anode as a measurable electrical pulse. This pulse, proportional to the intensity of the original light, is processed to determine the energy and location of the gamma rays, enabling accurate imaging in nuclear medicine.

2.2. The Role of Collimators
Collimators are integral to gamma cameras, restricting the field of view to prevent unwanted radiation from reaching the detector. They are made of high-density materials like lead and come in various designs:
Pinhole Collimators: Used for small organs, offering magnified images.
Converging Collimators: Magnify small regions by focusing radiation onto the detector.
Diverging Collimators: Minimize large organs for imaging.
Parallel-hole Collimators: Provide a one-to-one representation of the organ.
Each design serves specific clinical needs, balancing sensitivity and resolution.

2.3. Pulse Height Analyzer
Gamma rays of varying energies can originate from a single radionuclide, multiple radionuclides, or as a result of gamma-ray scattering within the source or detector. Consequently, the pulses produced by the amplifier may differ in magnitude. A pulse height analyzer (PHA) is a device designed to count only those pulses that fall within pre-selected voltage intervals or channels, while rejecting all others.
2.4. Basic Performance Characteristics of Imaging Systems
The count rate measured for a radioactive sample reflects the rate of decay of atoms within the sample, but determining the actual activity of the sample requires accounting for several determinate errors. The expression for the relationship between the sample activity and the measured count rate:

2.4.1. Background Count Rate
Residual radiation detected even without a radioactive sample, originating from cosmic rays, natural radioactive materials, or nearby sources, can be minimized using methods such as lead shielding, pulse height analyzer (PHA) circuits, and coincidence or anti-coincidence circuits. Coincidence circuits detect radiation events that occur simultaneously in multiple detectors, while anti-coincidence circuits exclude such simultaneous events, thereby improving measurement accuracy by reducing false signals.
2.4.2. Resolving Time
The time interval required for a detector to record successive events independently. Measured using oscilloscopes, it affects the system's ability to handle high event rates.
2.4.3. Detector Efficiency
The proportion of radiation particles or photons that interact with the detector. Efficiency depends on detector size, material, and radiation type, with NaI(Tl) crystals offering varying efficiency for gamma and X-rays.
2.4.4. Geometry Correction
Accounts for the fraction of emitted radiation reaching the detector, influenced by the solid angle subtended by the detector and its distance from the source.
2.4.5. Fractional Emission and Scattering
Factors like scattering in the source container or shielding can redirect radiation to the detector, potentially altering measurement accuracy.
2.4.6. Air and Window Absorption
Radiation attenuation occurs due to materials between the source and detector, including air, covering layers, and detector windows.
2.4.7. Self-Absorption
Radiation absorbed within the sample itself limits the accuracy of disintegration-rate measurements, particularly in solid samples.
2.5. Performance Parameters of Gamma Cameras
The quality and accuracy of gamma camera imaging are determined by the following performance parameters:
2.5.1. Spatial Resolution:
Measures the ability of the gamma camera to distinguish between two close points of radioactive distribution.
Determined by intrinsic resolution (related to the detector and electronics), collimator resolution (dependent on collimator design), and scatter resolution (affected by tissue interactions).
2.5.2. Intrinsic Resolution:
Related to detector performance and electronics.
Improves with thinner detectors and higher gamma-ray energy.
Narrow PHA (Pulse Height Analyzer) windows reduce scattered radiation, enhancing resolution.
Most modern gamma cameras have an intrinsic resolution of approximately 4 mm full width at half maximum (FWHM) for 140 keV photons emitted by 99mTc.

2.5.3. Collimator Resolution:
Affects the spatial resolution and depends on collimator hole size, length, and design.
Long, narrow holes enhance resolution, while the source-to-collimator distance should be minimized for optimal results.
Septal penetration by gamma rays can blur images, requiring careful energy selection (50–300 keV is preferred).



(A: High sensitivity parallel hole, B: Diverging, C: All-purpose parallel hole, D: Converging, E: High Resolution Parallel Hole, F: Pinhole)
2.5.4. Scatter Resolution:
Scattered radiation from patient tissues or other sources can degrade image clarity by producing acceptable pulses in the PHA.
Scatter resolution depends on the scattering medium, source configuration, and PHA settings.
2.5.5. Sensitivity:
Refers to the ability of the camera to detect gamma photons efficiently.
Collimators with shorter holes (high sensitivity) improve photon detection but at the cost of reduced spatial resolution.
2.5.6. Uniformity:
Ensures consistent response across the detector, critical for accurate imaging.
2.5.7. Contrast:
Describes the ability to distinguish between different levels of radioactivity within the image, influenced by scatter and detector efficiency.
Modern gamma cameras incorporate advanced computational technologies, allowing real-time dynamic imaging and enhanced diagnostic accuracy. As research continues, these systems evolve, promising even better spatial resolution and versatility in medical applications.
3. Spatial Resolution in Medical Imaging
Spatial resolution is a critical performance parameter in medical imaging, directly influencing the clarity and detail of diagnostic images. In nuclear medicine, where gamma cameras play a pivotal role, evaluating and enhancing spatial resolution ensures accurate and reliable imaging. This article explores the techniques and factors affecting spatial resolution in gamma cameras.
3.1. Evaluating Spatial Resolution: Techniques and Tools
Gamma cameras undergo rigorous quality control to ensure optimal performance. The spatial resolution is assessed using various techniques, including bar phantoms, the Line Spread Function (LSF), and the Modulation Transfer Function (MTF).
3.1.1. Bar Phantoms
What are Bar Phantoms? These are tools used to qualitatively evaluate spatial resolution by visually inspecting the image obtained. Thin lead bars are placed over the gamma camera detector, and their visibility determines the device's resolution. Over time, aging devices may struggle to capture the details of thin bars.

The Process: A flood source (commonly 57Co) is placed over the bar phantom, and the resulting image is analyzed. While effective, this method is qualitative and lacks precise measurements.
3.1.2. Line Spread Function (LSF)
How Does it Work? A radioactive line source is placed in the gamma camera’s field of view. A computer collects the data, generating a bell-shaped curve where the Full Width at Half Maximum (FWHM) of the curve quantifies spatial resolution.
Limitations: Scatter and septal penetration effects may not be fully accounted for, potentially impacting the accuracy of LSF measurements.
In Figure 3.2, the Line Spread Function of a gamma camera with a low-energy all-purpose parallel-hole collimator was obtained in air at different distances using a 99mTe-line source.

3.1.3. Modulation Transfer Function (MTF)
A Quantitative Measure: MTF evaluates the imaging system’s ability to depict different spatial frequencies. It considers scatter and septal penetration, providing a comprehensive measure of spatial resolution.

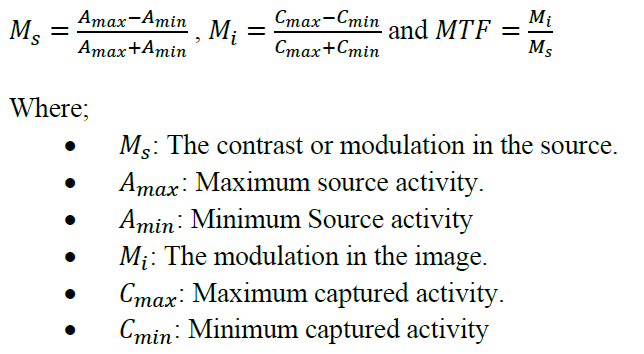
Key Insights: At low spatial frequencies, most systems perform well (MTF ≈ 1). However, performance varies significantly at higher frequencies, where finer details need to be resolved. As shown in Figure 3.4, at higher frequencies, system A provides the best resolution, followed by system B and system C in that order.

3.2. Factors Affecting Spatial Resolution
3.2.1. Detector Characteristics
Thinner detectors enhance resolution by minimizing scatter but reduce sensitivity.
Detector efficiency and geometry also play significant roles in resolution.

(A: High sensitivity parallel hole, B: Converging, C: All-purpose parallel hole, D: Diverging, E: High Resolution Parallel Hole, F: Pinhole)
3.2.2. Collimator Design
Hole Size and Shape: Smaller, longer holes improve resolution but may reduce sensitivity.
Distance to Source: Collimators perform best when the object is close to the detector.
3.2.3. Image Quality Components
3.2.3.1. Unsharpness
Geometric Unsharpness: Caused by the imaging process's geometry, influenced by factors like source size and distances between the source, patient, and detector.

(A: Minimal unsharpness with a focal spot of infinitely small source size. B: Geometric unsharpness due to a focal spot of finite source size. C: Geometric unsharpness caused by moving the object farther from the receptor. D: Geometric unsharpness, , caused by moving the object farther from the source. )
Subject Unsharpness: Occurs when structures in the object lack sharp boundaries due to gradual variations in composition or shape.

Motion Unsharpness: Results from motion during imaging, either voluntary or involuntary, spreading boundaries across the image and causing blur.
Receptor Unsharpness: Introduced by the image receptor, with its resolution affecting the sharpness of displayed images.
3.2.3.2. Contrast
Intrinsic Contrast: Depends on differences in physical or physiological properties of tissues, such as activity distribution or density differences.
Imaging Technique: Contrast can be enhanced through careful selection of imaging parameters.
Contrast Agents: Substances introduced into the body to improve contrast in specific regions (e.g., iodine for kidneys, barium for the gastrointestinal tract).
Receptor Contrast: Influenced by the image receptor's properties and resolution, affecting the ability to distinguish abnormalities.
3.2.3.3. Image Noise
Structure Noise: Irrelevant structural information in the image, such as rib shadows in lung radiographs.
Radiation Noise: Non-useful radiation, including scattered radiation, that reduces image clarity and contrast.
Receptor Noise: Variations in the sensitivity of detectors or contamination that adds noise to the image.
Quantum Mottle: Noise caused by the finite number of radiation particles forming the image, reduced by increasing exposure but at the cost of time and dose.
3.2.3.4. Image Distortion and Artifacts
Image Distortion: Unequal magnification of structures due to imaging geometry.
Artifacts: Features in the image that do not correspond to the original object, caused by factors like motion, improper settings, or system limitations.
3.2.3.5. Error, Accuracy, and Precision
Systematic Errors: Constant deviations from true values due to malfunctioning instruments or conditions.
Random Errors: Variable deviations caused by fluctuations in experimental conditions, such as high-voltage instability.
Accuracy and Precision: Accuracy reflects how closely measurements align with the true value, while precision measures reproducibility.
Spatial resolution is a cornerstone of effective nuclear imaging. By leveraging advanced assessment techniques like MTF and improving detector and collimator designs, medical imaging systems can deliver clearer, more accurate images. Continued innovation and stringent quality control in gamma cameras promise better diagnostic capabilities, shaping the future of healthcare.
4. Harnessing the Power of Computers in Nuclear Medicine Imaging
The integration of computers into nuclear medicine has revolutionized the way radiopharmaceutical data is acquired, processed, and analyzed. From the mid-1960s to today, the use of digital technology has enabled faster, more accurate imaging and diagnosis. This section explores the role of digital data acquisition, image processing, and advanced computer applications in nuclear medicine.
4.1. Digital Data Acquisition: The Backbone of Modern Imaging
In nuclear medicine, the signals from gamma cameras are analog in nature, requiring digitization for further processing. This involves:
Analog-to-Digital Converters (ADCs): Convert gamma-ray signals into discrete digital values for storage and manipulation.
Digital-to-Analog Converters (DACs): Convert digital data back into analog forms for display on video monitors.
4.2. Matrix Size and Pixel Depth:
Digital images are stored in matrices, where each pixel represents a specific location on the gamma camera detector. The spatial resolution of the image depends on the matrix size and pixel depth:
Larger matrices improve resolution but require more memory and processing power.
Optimal pixel size, approximately FWHM/3, ensures no significant resolution loss.
The matrix size is in powers of two, such as 32x32, 64x64, ... up to 512x512. The reason for using powers of two is to accelerate Fast Fourier Transform (FFT) operations on computers and to simplify memory management. The pixel depth is either 8-bit (byte) or 16-bit (word).

4.3. Data Acquisition Modes
Nuclear medicine employs two primary modes of data acquisition: Frame Mode and List Mode.
4.3.1. Frame Mode
Commonly used in static, gated, and dynamic studies.
Data are stored as matrices, with each pixel accumulating counts over time or a preset total.
Zooming can reduce pixel size and improve resolution within a focused field of view.
As shown in Figure 4.2, if the signal is within the energy window, the ADC digitizes the X and Y spatial coordinate signals to produce discrete numbers that are used to address a pixel in the computer memory.

4.3.2. List Mode
Stores X, Y coordinates with time stamps for each event, allowing flexible post-acquisition processing.
Used for gated cardiac studies, where arrhythmic cycles can be excluded during reformatting.
As shown in Figure 4.3, in order to produce digitized images, the list mode data must be formatted into frames of matrix data. This mode is frequently used in areas where the quality of imaging is dependent on timing, such as in cardiac health.

4.4. Advanced Image Processing
Computers enable sophisticated techniques for processing and enhancing nuclear medicine images:
Spatial Filtering: Reduces quantum mottle and structural noise for clearer images.
Static Studies: Focused data acquisition from a single region of interest.
Dynamic Studies: Sequential imaging to track tracer kinetics, essential for functional studies like cardiac and renal scans.
Gated Studies: Synchronize data acquisition with cardiac cycles to calculate heart function metrics, such as ejection fraction.
Image Display and Enhancement: Digital images are converted into analog for display. Techniques like windowing improve contrast by focusing on specific image regions.
Superimposition and Subtraction: Functional images from nuclear medicine (e.g., PET) can be overlaid on anatomical images (e.g., CT or MRI) for comprehensive analysis. Subtraction techniques remove background activity or isolate specific features.
Archiving and Networking: Picture Archiving and Communication Systems (PACs) allow efficient storage and retrieval of patient data. PACs support networking across multiple modalities, facilitating integrated diagnostics and streamlined administrative workflows.
5. Computed Tomography (CT) in Medical Imaging
Computed Tomography (CT) represents one of the most groundbreaking innovations in medical diagnostics. By overcoming the limitations of classical radiology, CT scans provide detailed cross-sectional images of the body, aiding in the diagnosis and treatment of various conditions. This section explores the history, principles, and advancements in CT technology.
5.1. The Birth of CT: A Milestone in Medical Imaging
5.1.1. Invention and Early Development:
CT was introduced in the early 1970s by Godfrey Hounsfield. The first clinical scan occurred in 1971 at Atkinson Morley's Hospital, producing an image with an 80x80 matrix. Today’s scanners achieve resolutions of up to 1024x1024, completing scans in fractions of a second.
5.1.2. Nobel Prize Recognition:
In 1979, Hounsfield and Allan Cormack received the Nobel Prize for their contributions to the development of CT technology.
5.2. How CT Works (X-ray Radiation Imaging)
CT imaging reconstructs cross-sectional images from multiple X-ray projections. This is achieved through the interaction of electromagnetic radiation with body tissues, governed by the exponential attenuation law:


By rotating the X-ray source and detectors around the patient, a detailed map of attenuation coefficients is obtained, converted into grayscale images.

Hounsfield Units (HU) standardize these measurements, where:
Air = -1000 HU
Fat = -120 to -60 HU
Water = 0 HU
Bone = +1000 HU
As in Figure 5.3, Computed tomography collects a series of views and reconstructs the corresponding image from them. Each sample within a view represents the sum of the image values along the ray that intersects that sample. In this example, the image consists of a small pillbox surrounded by zeros. Although only three views are illustrated here, a typical CT scan involves hundreds of views taken at slightly varying angles.


5.3. Generations of CT Scanners
CT technology has evolved through several generations:
First Generation: Single pencil beam and NaI(Tl) detectors, requiring linear and rotational movements.

Second Generation: Fan-shaped beams with multiple detectors, reducing rotation steps.

Third Generation: Arc-shaped detector arrays rotating with the X-ray source, improving speed and resolution.

Fourth Generation: Fixed detectors forming a complete ring, allowing continuous data acquisition.

Fifth Generation: Designed for cardiac imaging, using electron beams for ultra-fast scanning.

Sixth Generation: Helical CT with slip-ring technology, enabling continuous rotation and reducing scan times.

5.4. Applications of CT in Medicine
CT has become indispensable in various medical fields:
Oncology: Detecting tumors, monitoring treatment efficacy.
Cardiology: Evaluating heart function and detecting blockages.
Trauma Care: Identifying internal injuries and bleeding.
Neurology: Diagnosing brain abnormalities such as strokes and tumors.
5.5. Challenges and Limitations
Despite its advantages, CT imaging faces challenges:
5.5.1. Beam Hardening Artifacts:
Beam hardening occurs when lower-energy X-rays in a polychromatic beam are absorbed more readily than higher-energy X-rays as the beam passes through an object. This leads to artifacts such as dark streaks along areas of high attenuation and bright streaks elsewhere, as well as a reduction in Hounsfield units, known as the cupping artifact. Modern scanners mitigate this effect using beam-hardening correction algorithms.
As shown in Figure 5.11, beam hardening in simulated scans is demonstrated as follows: (A–D) Simulated scans without beam hardening and (E–H) scans with beam hardening. Dark streaks appear along the lines of greatest attenuation, while bright streaks are visible in other directions. Scatter also produces artifacts that resemble these patterns. Furthermore, a subtle decrease in Hounsfield units is observed just beneath the surface of the 'abdomen,' caused by beam hardening. This effect, known as the cupping artifact, is corrected using the beam-hardening correction integrated into modern scanners.

5.5.2. Partial Volume Effects:
The partial volume effect occurs when a CT scan divides anatomical structures into small, adjacent boxes during slice selection and reconstruction. These boxes may contain a mix of different tissues, such as bone, muscle, and blood vessels, resulting in an averaged CT number that does not accurately represent any single tissue. This effect can introduce false details into the image and compromise the reliability of the absolute CT number scale. It is a limitation in all tomographic imaging modalities, and the only way to mitigate it is by increasing spatial resolution so that each reconstructed box primarily contains one tissue type.
5.6. Summary
CT imaging is based on the principle that the intensity of an X-ray beam decreases as it passes through tissue, depending on the total attenuation of the tissue along its path. During a CT scan, a series of views is acquired, with all detectors simultaneously measuring the intensity of the beam for each view over a short period of time. Each set of these simultaneous measurements is referred to as a projection. The fundamental goal of CT imaging is to reconstruct a detailed image of the scanned area from these projections.

6. Positron Emission Tomography (PET)
Positron Emission Tomography (PET) has emerged as a transformative technology in medical imaging, offering unparalleled insights into molecular and cellular processes. By visualizing physiological functions and metabolic activity, PET enables earlier disease detection and more precise treatment planning compared to conventional imaging methods like CT and MRI.
6.1. What is PET?
Developed from advancements in nuclear medicine, PET relies on the detection of annihilation photons produced during positron decay. This imaging modality is unique in its ability to:
Map Functional Morphology: Visualizing how organs function rather than their structural appearance.
Detect Cellular Activity Changes: Identifying abnormalities before anatomical changes occur.
6.2. How PET Works
6.2.1. Radiopharmaceutical Injection
A biologically active molecule tagged with a positron-emitting isotope (e.g., 18F-FDG) is introduced into the body. These tracers accumulate in areas of high metabolic activity, such as tumors.
6.2.2. Positron Emission and Annihilation
The tracer emits positrons, which collide with electrons in the surrounding tissue.
This collision produces two photons traveling in opposite directions, which are detected by PET scanners.
6.2.3. Data Collection and Reconstruction
Detectors measure the photons' travel paths (Lines of Response, LORs).
A computer reconstructs these signals into detailed 3D images using algorithms like filtered back projection.
6.3. Important Definitions
6.3.1. Radiopharmaceutical
A radiopharmaceutical is a specialized drug composed of a radioisotope attached to an organic molecule, designed for either diagnostic or therapeutic purposes. The organic molecule targets specific organs, tissues, or cells, while the radioisotope emits radiation detectable by scanners. These substances must be non-toxic, stable enough to reach the target site, and soluble to cross cell membranes. Most radiopharmaceuticals combine a carrier molecule with a radionuclide, which is carefully selected for its chemical and physical properties. Due to their short half-lives, many preparations, such as technetium-based and PET agents, have limited shelf lives, often requiring use within hours of production. Ensuring radiochemical stability and minimizing impurities are critical to their effectiveness.
Ideal Properties of a Radiopharmaceutical:
Short physical half-life for minimal radiation exposure.
Eliminated from the body with an effective half-life matching the examination time.
Emits pure gamma rays through isomeric transition.
Gamma rays should be monoenergetic (~140 keV).
High specific activity for efficient targeting.
Quickly and largely localizes at the target site.
Decays into a more stable daughter nucleus.
Easily and effectively attaches to chemical compounds at room temperature.
Affordable per patient dose.
Easy to produce or store at the hospital site.
6.3.1.1. Diagnostic Radiopharmaceuticals:
These agents are designed to provide essential insights into organ function and physiological processes. By emitting gamma rays of sufficient energy to escape the body, they allow for non-invasive imaging. The isotopes used for diagnostics, such as those for brain blood flow or kidney function, have short half-lives to ensure they decay soon after imaging, minimizing radiation exposure. Frequently used diagnostic radiopharmaceuticals are listed below:
Nitrogen-13 (9.9 min half-life): Used as 13N-ammonia for PET imaging to assess myocardial perfusion under stress or rest in coronary artery disease patients.
Chromium-51 (27.7 days): Labels red blood cells and helps diagnose gastrointestinal protein loss and pernicious anemia.
Dysprosium-165 (2.33 hours): Applied as aggregated hydroxide for arthritis treatment (synovectomy).
Fluorine-18 (109.8 min): As FDG, detects tumors, metastasis, and studies glucose metabolism in the brain, heart, and tumors.
Iodine-131 (8 days): Diagnoses thyroid gland function and size; also evaluates kidney function.
Iodine-125 (60 days): Assesses kidney filtration rates and diagnoses deep vein thrombosis.
Iron-59 (44.5 days): Used for iron metabolism studies in the spleen.
Oxygen-15 (122 seconds): Tracer for studying oxidation processes, tissue water content, and regional blood flow.
Potassium-42 (12.36 hours): Evaluates exchangeable potassium in coronary blood flow.
Rubidium-86 (18.64 days): Measures myocardial blood flow.
Selenium-75 (120 days): Studies digestive enzyme production using seleno-methionine.
Sodium-24 (15 hours): Examines sodium exchange processes.
Xenon-133 (5.2 days): Used for pulmonary ventilation studies.
Gallium-67 (78.3 hours): Tumor imaging and detecting inflammatory lesions.
Indium-111 (67.2 hours): Helps image neuroendocrine tumors with pentetreotide.
Holmium-166 (26.8 hours): Investigated for liver tumor diagnosis and treatment.
Strontium-89 (50.56 days): Targets cancer sites to relieve bone pain in palliative care.
Thallium-201 (73.1 hours): Diagnoses coronary artery disease and heart muscle damage; locates low-grade lymphomas.
Carbon-11 (20.4 min): Labels red blood cells for pulmonary function studies.
Radionuclides suitable for PET are the short lived (half-lives on the order of seconds, minutes, or a few days) positron emitting isotopes:

6.3.1.2. Therapeutic Radiopharmaceuticals:
In contrast, therapeutic radiopharmaceuticals use isotopes that emit short-range particles (alpha or beta) to destroy cancer cells or relieve pain, especially in cases like bone cancer or arthritis. These particles cause localized damage due to their high energy transfer over small distances.
Beta Particle Emitters: Commonly used in clinical practice for their balance of range and energy transfer, making them suitable for various tumor sizes.
Alpha Particle Emitters: Preferred for precise, localized damage, affecting only a few cell diameters. However, their clinical use is limited due to challenges in production and short half-lives. Only isotopes like Astatine-211 and Bismuth-212 are currently viable for therapy.
The selection of the isotope and particle type depends on tumor size, distribution, and the radiopharmaceutical's pharmacokinetics, ensuring targeted and effective treatment.
6.3.2. Decay Constant

6.3.3. Uptake
Uptake is the process of absorbing and incorporating substances into a living organism.

6.3.4. Activity Measurement of Radionuclides
The activity of a radionuclide produced by the irradiation of a target material with charged particles in a cyclotron or nuclear reactor is given by:

6.3.5. Coincidence Circuit
A coincidence circuit in Positron Emission Tomography (PET) is used to detect the simultaneous arrival of two gamma photons produced during an annihilation event when a positron collides with an electron. These photons travel in opposite directions and are detected by paired detectors. The coincidence circuit ensures that only photon pairs detected within a very short time window (e.g., 15 nanoseconds) are considered part of the same annihilation event. This process helps identify the line along which the annihilation occurred, enabling the reconstruction of accurate tomographic images of the body.
As seen in Figure 6.1, if the time difference is approximately 15 ns, the signals originate from the same annihilation, and the coincidence circuit produces a signal.


6.3.5.1. Line of response (LOR):
In PET imaging, the Line of Response (LOR) refers to the straight path connecting two detectors that have detected a pair of gamma photons from an annihilation event. This line indicates where the annihilation likely occurred along that path.

6.4. Applications of PET
6.4.1. Cancer Diagnosis and Monitoring
PET excels in detecting cancers such as:
Breast, lung, and colorectal cancers.
Lymphomas and melanomas. By highlighting areas of high glucose metabolism, PET identifies tumors earlier than other imaging modalities.
6.4.2. Neurological Disorders
PET is vital in studying brain function and diagnosing conditions like:
Alzheimer's and Parkinson's diseases.
Epilepsy and schizophrenia. By measuring glucose consumption, PET maps regions of cerebral hypofunction or hyperactivity.

6.4.3. Cardiac Imaging
PET helps assess coronary artery disease and myocardial viability by differentiating between scarred and healthy heart tissue.

6.5. PET/CT: The Power of Fusion Imaging
The integration of PET with Computed Tomography (PET/CT) combines metabolic and anatomical imaging. This hybrid system:
· Pinpoints tumor location, size, and metabolic activity.
· Offers more accurate staging and treatment planning.
· Reduces interpretation errors caused by differences in patient positioning during separate scans.



6.6. Challenges and Future Prospects
· Short Half-Life of Tracers: Most PET isotopes (e.g., 18F, 11C) have short half-lives, necessitating proximity to production facilities.
· Image Artifacts: Scattered photons and random coincidences can reduce image contrast and accuracy.
As technology evolves, innovations in tracer chemistry, detector sensitivity, and AI-driven data processing promise to further enhance PET’s diagnostic capabilities.
7. Single Photon Emission Computed Tomography (SPECT)
Single Photon Emission Computed Tomography (SPECT) represents a significant advancement in nuclear medicine, providing a unique ability to visualize the functional processes of organs in three dimensions. By combining traditional gamma imaging with tomographic reconstruction techniques, SPECT offers insights that are both qualitative and quantitative.
7.1. The Evolution of SPECT Technology
7.1.1. Early Development:
SPECT’s roots trace back to the MARK IV system developed by Edwards and Kuhl. This prototype used a rectangular array of sodium iodide (NaI) detectors around the patient’s head.
7.1.2. First Commercial Device:
The Tomomatic-32, featuring 32 detectors, introduced SPECT to clinical applications.
Advancements in image reconstruction algorithms, such as filtered back projection (FBP), addressed early limitations like photon attenuation and scatter, establishing SPECT as a reliable clinical tool.

7.2. How SPECT Works
7.2.1. Radiopharmaceutical Administration
A tracer, tagged with a gamma-emitting isotope (e.g., 99mTc, 123I), is administered to the patient. The tracer localizes in specific organs or tissues, depending on the metabolic or functional activity.
7.2.2. Gamma Ray Detection
As the isotope decays, gamma rays are emitted. A gamma camera captures these rays, mapping variations in radiation intensity to create an image.
7.2.3. Data Acquisition and Reconstruction
Detectors rotate around the patient, collecting data over 180° (e.g., cardiac imaging) or 360° (e.g., full organ studies).
The data is reconstructed into 3D images, visualizing the radionuclide concentration in tissues.
7.3. Key Features of SPECT Imaging
7.3.1. Radioisotopes
Technetium-99m (Tc-99m) emits gamma rays at an energy of 140 keV and has a half-life of 6 hours, making it highly suitable for SPECT imaging due to its compatibility with gamma cameras and natural metabolic clearance times. It is derived from Molybdenum-99 and used in various applications, including kidney function assessment with Tc-DTPA, skeletal imaging, brain function analysis, and heart studies with specific compounds like isonitrile.

Iodine-123 (I-123) and Iodine-131 (I-131) are halogen isotopes used in biological and medical imaging, particularly for thyroid investigations. I-123 has a gamma energy of 159 keV and a half-life of 15 hours, making it ideal for diagnostic purposes but relatively expensive. In contrast, I-131, with a gamma energy of 364 keV and a half-life of 8 days, is less costly but challenging to collimate effectively due to its higher photon energy. These isotopes can replace stable iodine in natural biological molecules or mimic organic structures, allowing versatile diagnostic applications.
Xenon-133 (Xe-133) is a gamma-emitting inert gas with an energy of 81 keV and a half-life of 5.3 days. Its ability to cross the blood-brain barrier makes it useful for perfusion studies, particularly for measuring regional cerebral blood flow. Xe-133 is administered via inhalation or injection, and the clearance rate from the brain provides valuable information about blood supply to specific regions. It is cost-effective due to its production as a fission product.
7.3.2. Collimators:
Lead collimators filter gamma rays, ensuring only those aligned with the detector are captured, enhancing image clarity.
7.3.3. Photon Attenuation and Scatter:
Photon interactions within the body, such as Compton scattering, can degrade image quality. Correction techniques mitigate these effects, improving accuracy.
7.3.4. Partial Volume Effects:
Smaller structures may appear less active or larger than they are, necessitating recovery coefficients to adjust the measurements.
7.3.5. Center of rotation:
The center of rotation (COR) ensures proper image alignment in SPECT systems, and misalignment can cause artifacts like a "donut" effect, degrading image quality. Regular checks and adherence to manufacturer guidelines are essential to maintain accuracy.

7.4. Applications of SPECT
7.4.1. Cardiac Imaging:
SPECT is extensively used for evaluating myocardial perfusion and diagnosing coronary artery disease.
7.4.2. Neurology:
Mapping cerebral blood flow helps in diagnosing conditions like stroke and dementia.
7.4.3. Bone Scanning:
99mTc-labeled phosphonates detect abnormalities in skeletal structures, aiding in diagnosing fractures, infections, or metastases.
7.5. The Rise of SPECT/CT Hybrid Systems
7.5.1. Integration of Function and Anatomy:
Introduced in 1999, SPECT/CT combines functional imaging from SPECT with anatomical detail from CT. This synergy:
Improves localization of abnormalities.
Enhances diagnostic confidence.

7.5.2. Applications:
From detecting tumors to evaluating organ functionality, SPECT/CT provides comprehensive diagnostic insights.

7.6. Challenges in SPECT Imaging
7.6.1. Attenuation and Scatter:
Variations in tissue density can distort photon counts. Correction methods, such as phantom calibration, are essential.
7.6.2. Artifacts:
Errors in center-of-rotation alignment or collimator positioning can degrade spatial resolution.
7.6.3. Image Reconstruction Limitations:
Algorithms must balance resolution and computational efficiency.
8. Conclusion: Bridging the Past, Present, and Future of Medical Imaging
Medical imaging has come a long way from the discovery of X-rays to the sophisticated modalities of today, such as CT, PET, and SPECT. Each technology brings unique capabilities to the table, enabling clinicians to visualize the human body not just anatomically but also functionally and metabolically. This convergence of innovation and precision has transformed diagnostics, treatment planning, and patient care.
8.1. Key Takeaways:
8.1.1. Revolutionary Progress
The evolution of imaging technologies like CT and SPECT addressed the limitations of classical radiology, providing high-resolution cross-sectional images.
PET introduced the ability to visualize molecular and metabolic activity, identifying diseases at their earliest stages.
8.1.2. Integration of Modalities
Hybrid systems such as PET/CT and SPECT/CT have revolutionized diagnostics by combining anatomical and functional imaging. These systems provide unparalleled accuracy, guiding treatment decisions with greater precision.
8.1.3. Challenges and Innovations
Despite advancements, issues like photon attenuation, image artifacts, and the short half-life of tracers remain challenges. Ongoing research and technological improvements—such as AI-assisted image reconstruction—promise to overcome these barriers.
8.1.4. Personalized Medicine
With molecular imaging and advanced radiopharmaceuticals, imaging is moving toward personalized medicine. Physicians can now tailor treatments to the unique biological characteristics of each patient, improving outcomes and reducing side effects.
8.2. Looking Ahead
The future of medical imaging lies in the seamless integration of advanced imaging modalities, real-time data processing, and AI-driven analytics. As imaging becomes faster, more accurate, and less invasive, it will continue to play a pivotal role in enhancing our understanding of health and disease, ultimately improving the quality of life for patients worldwide.
By building on the foundations of past innovations and embracing future possibilities, medical imaging remains an indispensable tool in the hands of modern medicine.
9. References
Statikiewicz, M.A., Workbook for Radiation Protection in Medical Radiography, Mosby, St. Louise, 2010.
Prince, J.L., Links, J., Medical Imaging Systems and Signals, Prentice Hall, New Jersey, 2005.
Guerra, A.D., Ionizing Radiation Detectors for Medical Imaging, World Scientific Publishing Company, Singapore, 2004.
Martin, C.J., Medical Imaging and Radiation Protection, Vine House Distribut, New York, 2003.
Comments